Corrosion and rust in metals by Dr. Belen Evgenia
Corrosion and rust in metals by Dr. Belen Evgenia
The present day when the first metals were discovered, first the iron that was used for ancient weapons. After copper, brass, gold and silver, tin and lead, mercury and zinc, cobalt and magnesium and more…man is exposed to the phenomenon of corrosion and rust. Corrosion and in Hebrew fusion or consumption. An annoying phenomenon of weathering of the metals. which for many years man did not know how to respond to the phenomenon. Mainly to the accelerated weathering phenomenon in a marine environment in vessels and buildings near the sea, and later in a corrosive chemical atmosphere in the developing industrial plants, with the development of humanity.

With the development of chemistry and microbiology and the many studies that were required on the subject, it was proven that corrosion forms between metals and environmental conditions that react with the metals. This reaction causes the surface to be eaten away until a hole is filled with thin metals. If it is moisture in the air, salinity in the air, oxygen in the air. If it is a corrosive acidic atmosphere that attacks the metals. Corrosion attacks and crumbles and reduces the thickness of the metal, causing dents and holes and cracks. Corrosion causes metals to weaken and disintegrate over the years.
Corrosion and rust in metals
Overview Corrosion and rust in metals and methods of protection
Corrosion occurs with and without an electric current. With an electric current – wet corrosion, referring to an electrochemical reaction of the metal with a wet corrosive atmosphere with water. In this process the metal accumulates a low voltage anodic potential, and a higher positive cathodic potential. The voltage flow from high to low creates increased corrosion in the anodic area and thus the cathodic area is not attacked. (This is the trick behind galvanizing ferrous metals to obtain cathodic protection. Connecting the 2 types of metal creates a potential difference and in fact an electric current from the cathode to the anode. The anode rusts and the cathode does not. An electric current is created between the anode and the cathode like any household battery or car battery.) Without an electric current – dry corrosion, referring to corrosion caused by contact with the oxygen in the air or corrosive fumes emitted from various chemicals. These react with the metal without the formation of an electric field.
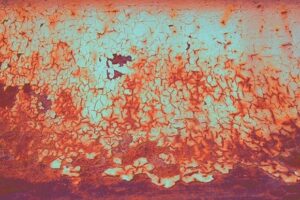
Corrosion in iron, its alloys and its products creates rust. In other non-ferrous metals, corrosion causes corrosion and corrosion. The FeO or Fe 2 O 3 rust that forms and accumulates on ferrous metals as it increases corrodes more and more until the destruction of the metal. A common method to delay the corrosion process is to coat the metal with hot or cold zinc, a process called galvanizing. This coating is first eaten by the corrosion and only after it is gone, the corrosion attacks the ferrous metal. A method that provides years of protection to the ferrous metal.
Overview Corrosion and rust in metals and methods of protection

Another and more common method is sand cleaning and epoxy and polyurethane paint systems, which seal the metal from contact with air and the atmosphere in which the metal is located. This prevents the development of rust. The epoxy epoxidan rust converter primer contains a corrosion inhibitor and rust converters that create a hard primer layer that prevents the development of rust. The top layer is Danberglus acrylic polyurethane paints that are resistant to outdoor conditions and seal the metal against the passage of air and moisture and the development of rust. To strengthen the colors from external mechanical hazards, the coating can be thickened with an intermediate layer of epoxy 80 Sulkot or Epoxy Ship Solkot Aluminum.

Basic epoxide converts rust

Epoxiden 80 Solcot

Denbergalos acrylic top
Denber Projects Company – Execution with over 30 years of experience
 English
English עברית
עברית Русский
Русский